Our science
-
 Soft Tissue Repair
Soft Tissue Repair
-
 Ischemic Stroke
Ischemic Stroke
-
 Retinal Vein Occlusion
Retinal Vein Occlusion
Soft Tissue Repair
(LBI-101)
Soft tissue loss occurs with aging, traumatic injuries, and surgical procedures such as tumor resection. Synthetic injections and implants remain the primary option for aesthetic wrinkle filling and breast reconstruction applications with both U.S. markets comprising over 2 billion procedures annually. Because synthetic implants do not fully restore form and function and can suffer from a fibrosis rejection response, fat grafting has emerged as an alternative procedure to provide natural soft tissue reconstruction. Autologous fat grafting procedures are growing in multiple clinical specialties for treating burn/scar, congenital craniofacial defects, lumpectomy and breast reconstruction, and facial rejuvenation. Unfortunately, fat grafting requires a surgical procedure to harvest the fat which limits wider use.
The Problem
Current methods for treating tissue loss rely primarily on synthetic materials that do not fully restore tissue form and function, or biological methods that are too costly or provide only temporary relief. In the case of superficial tissue loss caused by aging, loss of tissue elasticity and adipose under the skin leads to wrinkle formation. Current solutions to fill wrinkles are temporary and do not restore tissue form long term. For larger tissue defects created by surgeries such as mastectomy, synthetic implants and reconstructive surgery remain the standard of care. However there are still limited options for patients receiving lumpectomy. Fat grafting (moving fat from one place in the body to another) is a surgical alternative for reconstructing soft tissue. The number of fat grafting procedures is steadily growing but it requires a surgical procedure to harvest the tissue.
our solution
We are developing an off-the-shelf acellular adipose tissue biomaterial for reconstructing soft tissue. It combines the convenience of synthetic materials with the biology of natural tissue to stimulate repair and regeneration. Using native fat tissue, we process the matrix of adipose to create an injectable formulation that mimics fat grafting and is terminally sterilized.
Phase 1 clinical studies demonstrated safety and biocompatibility. Cells, including stem cells, migrated into the implant, inducing new tissue formation.
A Phase 2 dose escalation study increased the volume of injection and was used to treat patients with soft tissue loss after lumpectomy and mastectomy. Biological analysis revealed the ability of the product to rebuild tissue without scaring which is typically associated with the injection. The platform adipose technology can be processed into multiple forms for superficial or deep injections and generating solid implants. The injectable acellular adipose technology provides a biological reconstruction solution for permanently rebuilding soft tissue.

Development Status
Phase 2
Ischemic Stroke
(LBI-201)
Stroke represents one of the largest medical challenges facing our society today. In the U.S., someone has a stroke every 40 seconds and every four minutes someone will die as a result of a stroke. Stroke is the 5th leading cause of death in the U.S. and the 2nd leading cause of death worldwide. In the U.S. stroke kills approximately 130,000 people each year (roughly 1 in every 20 deaths).
Nearly 800,000 people have a stroke in the U.S. each year and approximately 87% are classified as ischemic (the result of a blood clot or blockage to one or more of the arterial vessels in the brain). Stroke is the leading cause of long-term disability in the U.S. Total direct medical costs related to stroke are expected to almost triple from $36.7 billion in 2015 to over $94.3 billion in 2035.
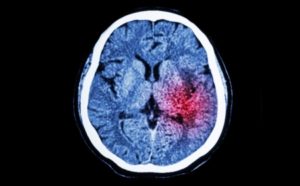

The Problem
Despite the significant medical burden associated with stroke, current treatment options remain extremely limited. Currently approved treatments for acute ischemic stroke include clot-dissolving drug (tPA) and catheter-based thrombectomy.
Whereas tPA is effective at dissolving small vessel blood clots, it is only effective at restoring blood flow in 15-20% of large arterial vessel occlusions. In contrast, catheter-based thrombectomy is highly effective at removing large vessel occlusions but is performed at less than 10% of hospitals nationwide due to the requirement for specialized facilities and trained medical staff.
our solution
Clinical studies have demonstrated that coupling the acoustic energy of transcranial ultrasound with conventional thrombolytic drug therapy (tPA) can significantly increase the rate of restoring blood flow in stroke patients with large vessel occlusions. Transcranial ultrasound generates acoustic streaming at the site of the blood clot, which in turn causes tPA to be refreshed at the surface of the occlusion. Through this streaming effect, it is believed that tPA can better access a greater number of binding sites on the clot surface thereby resulting in an accelerated and increased rate of clot dissolution.
To deliver the ultrasound, Longevity Biomedical has developed a non-invasive ultrasonic device that can be rapidly administered in the emergency room environment once the stroke diagnosis has been confirmed and IV tPA administered. Initiation of therapy does not require a trained sonographer or ultrasound technician and can easily be administered by an emergency room nurse or physician. The device is portable and designed to travel with those stroke patients that are determined to be viable candidates to undergo EMS transport to a Comprehensive Stroke Center for thrombectomy. By accelerating the rate of vessel recanalization in stroke patients that do not have immediate access to thrombectomy services, this novel therapy will address a significant treatment gap in existing stroke care.

Development Status
Phase 3 (NCT03519737)
Retinal Vein Occlusion
(LBI-001)
RVO is a chronic condition of the eye in which small blood clots form in the small veins that carry blood away from the retina. When this occurs, there is a hemorrhage of blood from the blocked vessels into the surrounding retinal tissue leading to swelling (macular edema) and the formation of new blood vessels (neovascularization). Left untreated, RVO typically leads to progressive deterioration of vision leading to permanent blindness. Risk factors for RVO include atherosclerosis, diabetes, and high blood pressure.
There are two primary types of RVO: Central retinal vein occlusion (CRVO) results when a clot forms in the main central retinal vein. Branch retinal vein occlusion (BRVO), which accounts for approximately two-thirds of all RVO cases occurs when the blockage forms in one or more of the smaller branch veins. RVO is one of the most common causes of vision loss worldwide and is the second most common cause of blindness from retinal vascular disease behind diabetic retinopathy. There are approximately 144,000 new cases of RVO in the U.S. each year.
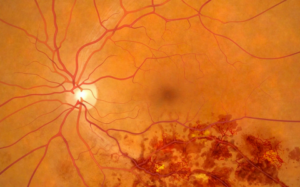

The Problem
Treatment options for RVO are limited. Current approved treatments include anti-VEGF drugs (Eylea, Lucentis) and laser photocoagulation. Anti-VEGF drugs work to halt the progression of neovascularization and require frequent and long-term injections directly into the eye. These injections are painful and must be repeated approximately every 1-2 months. Laser photocoagulation attempts to seal off the leaky blood vessels and halt further progression of neovascularization. Whereas these treatments have been shown to be effective at slowing the progression of the disease, they do not treat the underlying cause (i.e. a blood clot in one or more of the retinal veins).
As a result, most patients with RVO continue to suffer a progressive, deterioration of vision that significantly impairs their quality of life. A new therapy that can treat the actual cause of the disease, stop further progression and potentially eliminate the need for long-term maintenance therapy is desperately needed.
our solution
We have developed a new approach to the treatment of RVO that treats the root cause of the disease – namely the venous clot. The therapy combines intravenous administration of microspheres with non-invasive ultrasound delivered across the closed eyelid. The microspheres are approximately 3 microns in diameter and sufficiently small enough to pass through retinal vein occlusions. When traveling through the path of ultrasound, the microspheres undergo multiples cycles of expansion and contraction (cavitation). This cavitation produces mechanical forces in the vicinity of the blood clot to restore blood flow in the occluded vein. The ultrasound device is a small, portable instrument that can be easily administered in the eye clinic. By treating the underlying cause of the disease as opposed to the symptoms, this therapy has the potential to reduce or eliminate the need for chronic maintenance therapy and dramatically improve the quality of life for those patients inflicted by RVO.

Development Status
Phase 2b (IDE Cleared)

